Find a Device
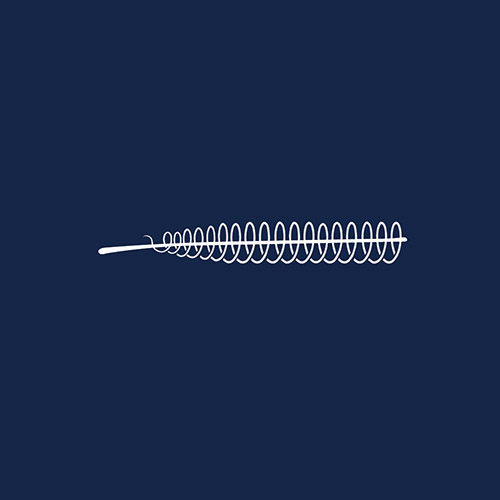
Essure Permanent Birth Control System is a prescription-only, non-hormonal form of permanent birth control for women. Although its manufacturer Bayer AG insists that it is 99.83% effective, tens of thousands of women have claimed the device has caused life-changing and life-threatening injuries. It is estimated that over 16,000 lawsuits have been filed against Essure.
As of July 20, 2018, Bayer has pulled Essure from American markets, with a plan to discontinue its sales completely after December 31 of this year.
Visit Drugwatch for more information.
Learn more from patient groups.
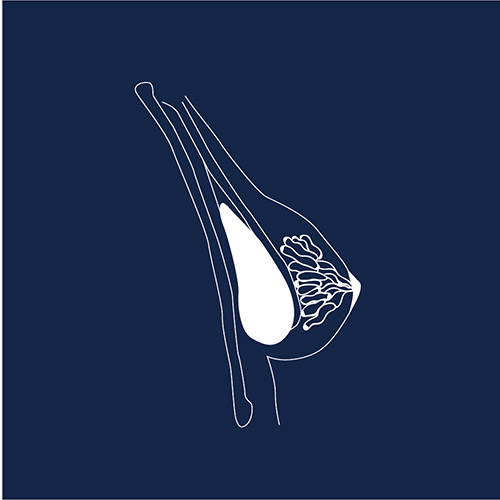
Breast implants are one of the most common medical devices, with over 300,000 women undergoing breast augmentation per year in the United States and more than 50,000 breast cancer patients getting breast implants after mastectomy. However, thousands of women have claimed adverse events with silicone and saline implants – from pain and infection, to severe autoimmune symptoms, and even a rare cancer of the immune system called ALCL.
Visit Breast Implant Info for more information.
Learn more from patient groups, such as Breast Implant Victim Advocacy and Breast Implant Illness and Healing.
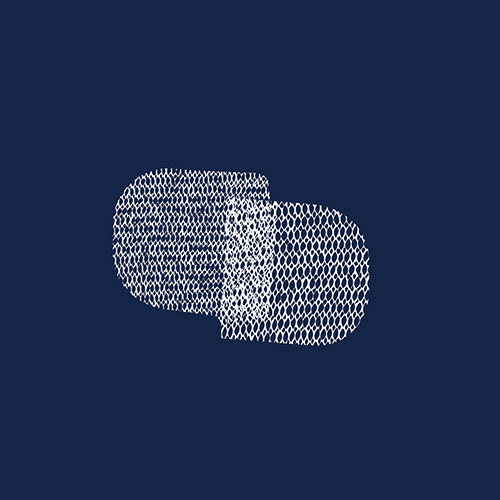
Transvaginal mesh is a net-like implant used to treat pelvic organ prolapse and stress urinary incontinence in women. It can cause various severe complications such as severe pain, nerve damage, tissue erosion and organ perforation. To date, more than 40,000 lawsuits have been filed against Boston Scientific, one of the device manufacturers.
Visit Drugwatch and Mesh Medical Device News Desk for more information.
Learn more from patient groups.
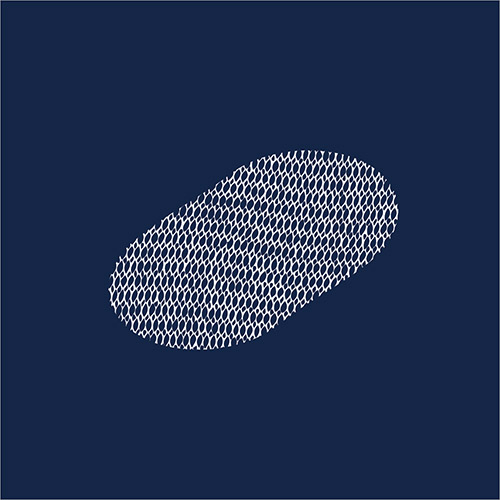
Surgical mesh is a flexible sheet made of plastic or organic materials used to reinforce or repair week tissue, with hernia repair surgery being the most common application. Patients have claimed complications that include infection, bowel obstruction, perforation of tissue and organs, migration, and adhesion (scar-like tissue that sticks together). It is estimated that over 3,000 lawsuit cases have been filed by people with hernia mesh injuries. Since 2005, over 200,000 mesh units have been recalled by different device manufacturers.
Visit Drugwatch for more information.
Learn more from patient groups.
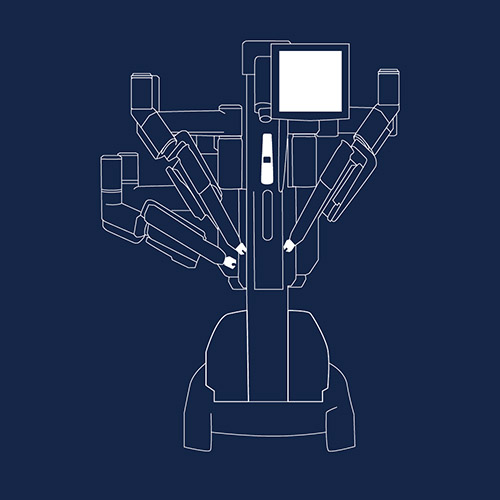
The da Vinci Surgical System has been used to help surgeons perform complex, minimally invasive surgeries on more than three million patients worldwide. Patients have suffered complications such as longer than usual operating and anesthesia times, device malfunction during procedures, injury to surrounding organs, infection, and scarring. Advocates claim underreporting of adverse events and lack of training have aggravated the issue.
Visit AARP and Drugwatch for more information.
Learn more from patient groups.
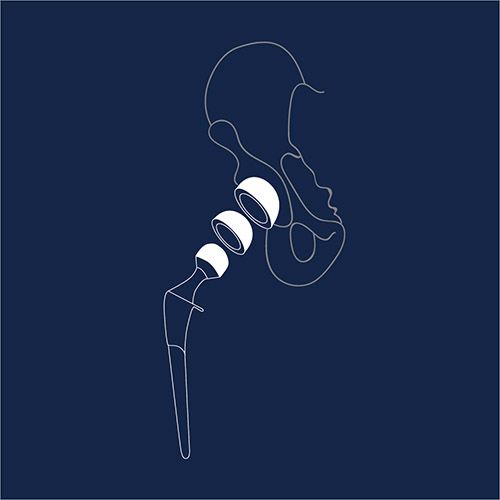
A hip replacement is a surgical procedure in which the hip joint is replaced by a prosthetic implant. Although a fairly common procedure, adverse reports have noted problems such as loosening of the implant, fractures, and avascular necrosis (“bone death”). In particular, implants using metals such as cobalt and chromium may present severe toxic risks, including metallosis, a type of metal poisoning. This condition causes muscle pain, tissue death, and bone damage. An estimated 14,000 lawsuitsagainst different manufacturers for metal-on-metal hip replacement devices are currently pending. Past metal-on-metal hip replacements overall settlement for expenses have included $56 million for Bionet, $1.4 billion for Stryker and a staggering $4 billion for Johnson & Johnson, DePuy’s parent company.
For more information please visit Dr. Stephen Tower’s updates and resources.
Visit Drugwatch for more information.
Learn more from patient groups.
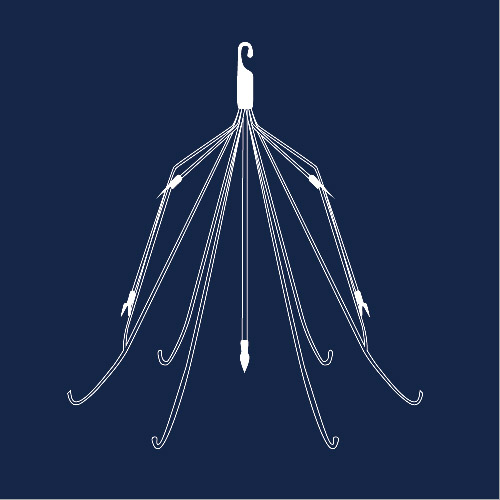
An inferior vena cava (IVC) filter is a small metal device designed to stop blood clots from traveling into the lungs, and possibly causing a pulmonary embolism. Known risks for the device include infection, excess bleeding, damage to the blood vessel, and a filter that travels to the heart or lungs, among others. After receiving almost 1,000 complaints regarding adverse reports between 2005 and 2010, the FDA began advising doctors to remove IVC filters once the thread of pulmonary embolism was no longer present. Unfortunately, many of the worst complications are experiences upon the removal of the filters.
Visit Drugwatch for more information.
To connect with patient groups.
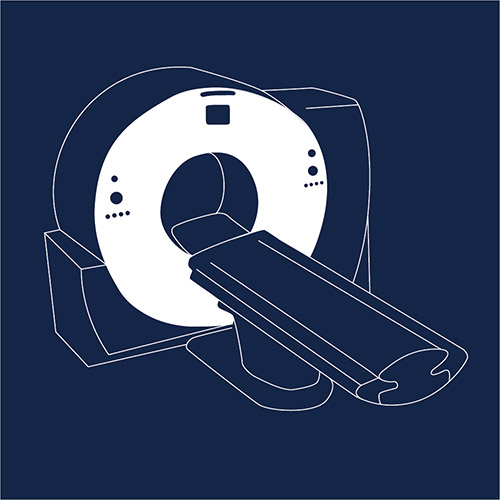
A computerized tomography (CT) scan combines a series of X-ray images taken from different angles around the body, using computer processing to create cross-sectional images inside the body. A CT scan is regularly used to help diagnose disease or injury as well as to plan treatments. It’s also often used to quickly examine patients who may have internal injuries from different types of trauma. Because CT scans gather more information than a regular X-ray, the body is exposed to a larger quantity of ionizing radiation. Although CT scans have many benefits that may outweigh any small potential risk, they are many times over prescribed due to financial incentives and fear of lawsuits. An American Medical Association study estimates that CT scan overuse causes 50,000 cancers every year.
Visit Safe Patient Project for more information.
Questions & Answers
Medical devices range from a bandage you would put on a scratch to a pacemaker that would be implanted in your body. They are defined as an instrument, apparatus, machine, implant, in vitro reagent, or such, intended for use in a diagnosis, a cure, a treatment, or a prevention of disease or condition. A key defining aspect is that a medical device does not achieve its primary intended purposes through chemical action nor by metabolization. Devices are classified as I, II, or III, with Class I presenting the lowest potential for harm (bandages, gloves, and hand-held surgical instruments), and Class III being those that usually sustain or support life, are implanted, or present higher risk of illness or injury (such as heart valves, breast implants, pacemakers, and birth control implants, such as Essure).
• How many procedures have you done?
• Have any patients had problems?
• Have you ever removed this device?
• Has this device ever been recalled?
• What are the risks and alternative treatments?
• Does this device have a black box warning or contraindications?
• What is the device made of, and what can be done if an adverse reaction to the materials occurs?
• Have you had any financial relationship with the manufacturer of the device you’re recommending?
Media

















