Over the past decade, nearly 70 million Americans have been implanted with medical devices.
I've been affected by a device I'm looking to learn more about devicesIn the last 4 years (2018-2021), they have been linked to the deaths of 37,135 patients.
What is a medical device?
Medical devices range from a bandage you would put on a scratch to a pacemaker that would be implanted in your body. They are defined as an instrument, apparatus, machine, implant, in vitro reagent, or such, intended for use in a diagnosis, a cure, a treatment, or a prevention of disease or condition. A key defining aspect is that a medical device does not achieve its primary intended purposes through chemical action nor by metabolization. Devices are classified as I, II, or III, with Class I presenting the lowest potential for harm (bandages, gloves, and hand-held surgical instruments), and Class III being those that usually sustain or support life, are implanted, or present higher risk of illness or injury (such as heart valves, breast implants, pacemakers, and birth control implants, such as Essure).
Examples
Learn more about these devices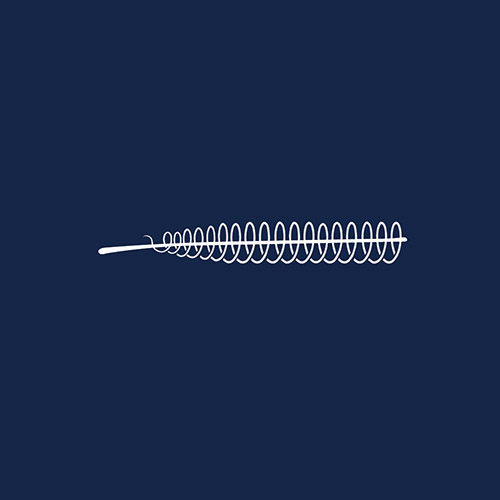
Essure
Essure Permanent Birth Control System was a prescription-only, non-hormonal implant advertised as the better alternative to tubal ligation for women who wanted to be sterilized. Two coils made of various metals and plastics were inserted into the fallopian tubes to block them, thereby preventing pregnancy. Although its manufacturer Bayer AG insists it’s safe and 99.83% effective, tens of thousands of women have claimed the device has caused life-changing and life-threatening injuries. After The Bleeding Edge documentary in 2018, the FDA placed sales restrictions on Essure, and Bayer “voluntarily” discontinued the product. Bayer announced in August 2020 that it was settling approximately 40,000 USA lawsuits for $1.6 Billion.
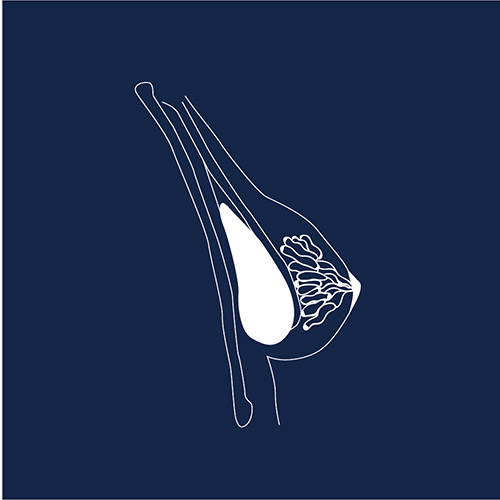
Breast Implants
Breast implants are one of the most common medical devices, with over 300,000 women undergoing breast augmentation per year in the USA and more than 50,000 breast cancer patients getting breast implants after mastectomy. However, thousands of women have claimed adverse events with silicone and saline implants – from pain and infection to severe autoimmune symptoms, and even a rare cancer of the immune system called BIA-ALCL: breast implant associated anaplastic large cell lymphoma. In 2019 the FDA requested a recall of all Allergan BIOCELL and Natrelle textured breast implants “to protect individuals from the increased risk of BIA-ALCL.”
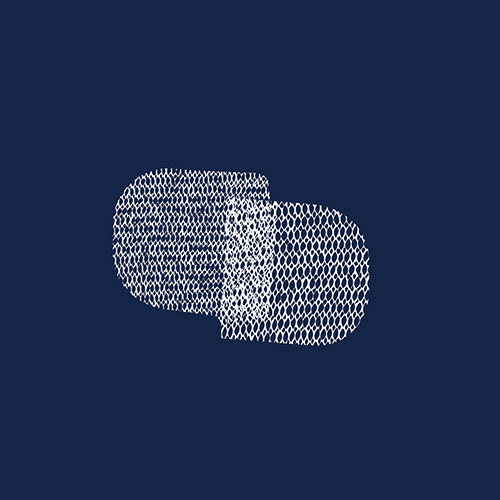
Transvaginal Mesh
Transvaginal mesh is a plastic polypropylene material used to treat pelvic organ prolapse (POP) and stress urinary incontinence in women. It has caused problems such as severe pain, nerve damage, tissue erosion and organ perforation. In April 2019, the FDA halted sales of transvaginal mesh for POP. Thousands of lawsuits have been filed against Boston Scientific for their transvaginal mesh device, and in April 2022 an appeals court ordered Johnson & Johnson to pay $302 million to the state of California for deceptive marketing of pelvic mesh implants for women. Another verdict landed in May 2022 against C.R. Bard in the second bellwether trial, and litigation is ongoing.
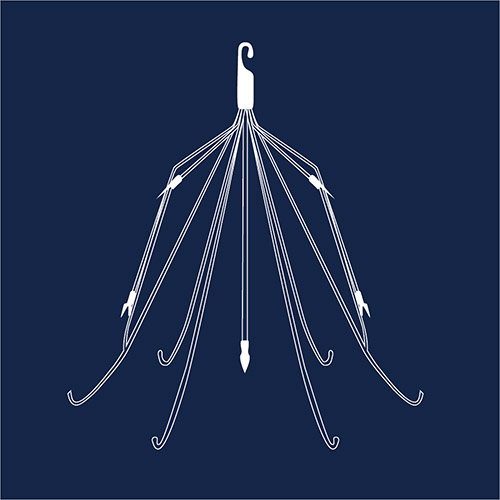
IVC Filters
An inferior vena cava (IVC) filter is a small metal device, usually made from Nitinol or other flexible metals, which is designed to stop blood clots from traveling into the lungs and possibly causing a pulmonary embolism. Adverse event reports for these devices show problems such as infection, allergic reactions, blood vessel damage and blockages. Several thousand lawsuits have been filed against manufacturers Cook Medical and Cordis. In 2018, a Texas jury awarded a firefighter $1.2 million against Cook Celect filters. There have also been three plaintiffs’ verdicts at trial in 2021, including a $3.3 million verdict in Wisconsin against Bard for their Meridian IVC Filter.
Surgical Mesh
Surgical mesh is a flexible sheet, usually made of plastics like polypropylene or sometimes organic tissues, used to reinforce or repair week tissue. Hernia repair surgery is the most common application.
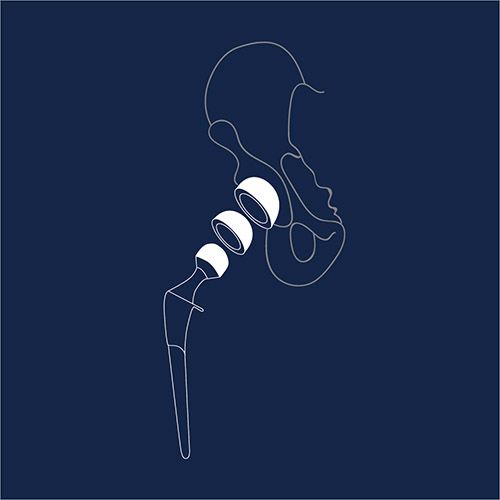
Metal-on-Metal Hip Replacements
A hip replacement is a procedure in which the hip joint is replaced by a prosthetic implant. Adverse event reports reveal problems such as loosening of the implant, fractures, and avascular necrosis (“bone death”).
da Vinci Surgical System
The da Vinci Surgical System helps surgeons perform complex, minimally invasive surgeries on more than three million patients worldwide. However, the device maker, Intuitive, is now subject to lawsuits for remotely shutting down the device during surgery and and forcing doctors to switch to riskier open procedures.
CT Scan
A computerized tomography (CT) scan combines a series of X-ray images taken from different angles around the body, using computer processing to create cross-sectional images inside the body.
Angie Firmalino founded Essure Problems, a 40,000+ strong patient advocacy group, that now brings together patients and activists together fighting for medical device safety.
Through her personal story and the forming of the E-Sisters, Angie has propelled forward the fight to remove Essure off the American market, with Bayer finally announcing on July 20, 2018 its plan to pull the device from American markets. You can watch Angie’s story in The Bleeding Edge documentary, on Netflix.
Questions & Answers
- Contact doctor to discuss the cause and next steps.
- Report an adverse event to the FDA (you may learn how to make a report with the Med Watch tutorial.)
- Research your device on the Find a Device page of this site.
- If you need to have a device removed and are in need of a physician to do so, you may contact the patient groups listed on the Find a Device page of this site.
- Research specific device recalls.
- Look up the device on the Find a Device section of this website
You may find out if any of your doctors have received money from device companies at ProPublica’s Dollars for Docs.
- You may research if your doctor has been sued by using the doctor’s name as a keyword in your search engine, along with key terms such as “malpractice,” “lawsuit,” or “complaint.”
- You may also use the AIM DocFinder, which will provide information on a physician’s licensing background and disciplinary information.
You can learn more about your surgeon’s experience before your procedure at ProPublica’s Surgeon Scorecard.
- Report medical device adverse events to the FDA via MedWatch.
- Encourage your hospital or practice to join a patient safety membership organization, such as the Institute for Healthcare Improvement/National Patient Safety Foundation’s Stand Up for Patient Safety Program.
- Follow the latest data on medical technology, adverse events, and recalls on the Device Events database.
- Become an Unbranded Doctor and join the National Physicians Alliance’s network committed to reducing the influence of pharmaceutical marketing in the field.
- Support the Medical Device Safety Act.
- Learn more about the Unique Device Identification (UDI) initiative and how it can help ensure device safety by reading the Pew Charitable Trusts’ report.

)
